Abstract
Multiple myeloma (MM) is characterized by the loss of critical mediators of immune surveillance and the activation of osteoclast. The transforming growth factor-beta (TGF-β) signaling pathway has been implicated in MM progression through promotion of catabolic bone remodeling, IL-6 secretion and Th17 T cell development, promoting osteolytic bone disease, immune suppression and MM progression. Here, we conducted preclinical studies in the syngeneic 5T3MM immunocompetent mouse model of MM to assess the single agent activity of vactosertib (Vacto, TEW-7197), a TGF-β type I receptor kinase inhibitor, and the potential to act synergistically with the third-generation immunomodulatory drug, pomalidomide (Pom). These data support the first clinic trial to evaluate this combination regimen in patients (pts) with MM (NCT03143985, open and recruiting).
Methods
Preclinical: Mice bearing 5T33MM cells expressing luciferase were treated with Vacto, Pom and the combination for 3 weeks, and evaluated for MM growth by bioluminescence imaging (BLI). Cellular and molecular assays were performed in human RPMI8226 and U266 as well as murine 5T33MM cells via apoptosis, real-time PCR and western blotting. Peripheral blood monoclonal protein concentration, M-spike, was measured by ELISA. Distal femur trabecular bone structure was assessed by 3D micro-CT. Phase I clinical trial: In this open-label study, pts with relapsed MM with at least two lines of therapies received escalating dosages of Vacto in combination with a standard dose of Pom (4 mg) without corticosteroids. The study was conducted as a modified Fibonacci 3 + 3 dose escalation design to assess safety, maximum tolerated dose, recommended phase 2 dose, and efficacy of Vacto/Pom combination in compared to historical control of Pom without corticosteroids (PFS-6: 20% in randomized Phase II study by Richardson et al. Blood. 2014). Vacto tablets are administered orally in 28-day cycles, taken once daily for 5 days followed by 2 days without treatment, repeated either for 6 cycles or until progression of disease or intolerable toxicity. Dosing was initiated at 60 mg and increased to 120 and 240 mg.
Results
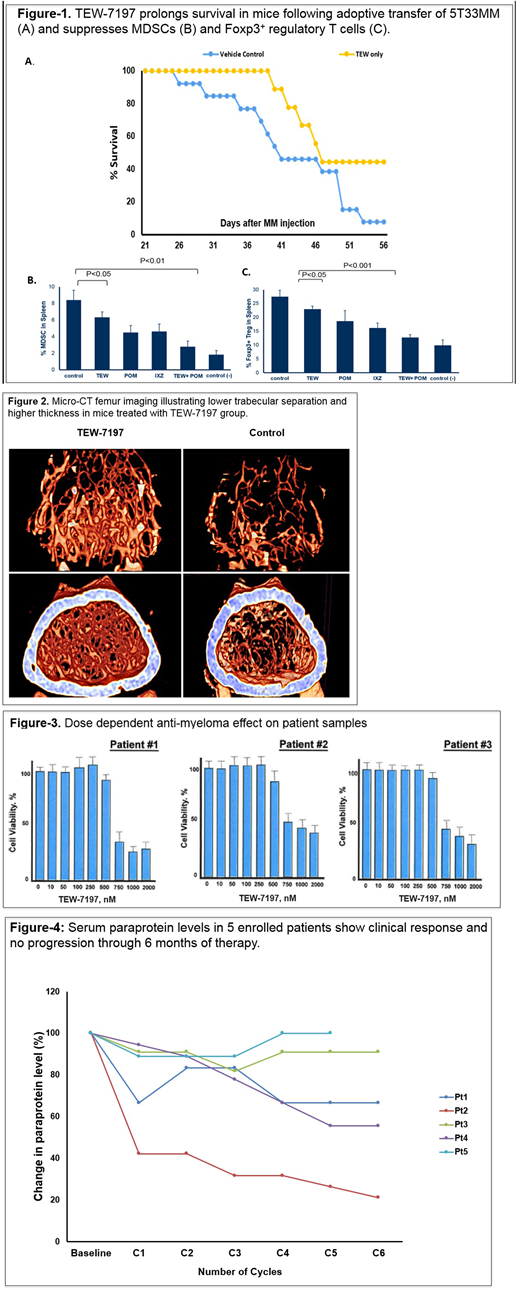
Preclinical: Vacto attenuated the growth and viability of human and murine MM cells by inducing apoptosis and inhibited TGF-β-induced activation of Smad2/3 in MM cells in vitro. In the 5T33MM preclinical model, single agent Vacto inhibited MM progression as measured by peripheral blood monoclonal protein concentration and BLI. Vacto also prolonged survival (Figure-1), prevented weight loss and increased trabecular bone thickness (Figure-2) in mice bearing MM. Vacto either alone or in combination with Pom also attenuated TGF-β activation of Smad2/3, reduced the expansion of CD11b⁺Gr-1⁺ myeloid derived suppressor cells (MDSCs) in the bone marrow and diminished the population of Foxp3⁺ regulatory T cells in the spleen (Figure-1). Vacto showed anti-myeloma activity in cells from three MM pts (Figure-3) at 700-750 nM in vitro. Phase I: As of 01 July 2018, 5 pts were enrolled; 3 pts received 60 mg daily and 2 pts received 120 mg daily. Patient demographics include: median age of 70 years (range: 64-77); 3-5 lines of prior therapy; ISS stage II or III; two pts had high risk cytogenetics including 17p deletion; three pts were refractory to bortezomib and four pts were refractory to lenalidomide. None of five enrolled pts developed any DLT or needed Vacto dose reduction .The most non-hematologic common adverse event (AE) was grade II fatigue and pain in one pt with no grade III or IV non-hematologic AEs. No pt experienced any progression of disease (PFS-6: 100%). Figure-4 illustrates fluctuations in disease markers at each cycle interval. Two pts had grade III hematologic AE, no grad IV hematologic AE.
Conclusions: Vacto has activity against myeloma in the syngeneic 5T33MM murine MM mouse model and in both human MM cell lines and primary patient MM samples. Remarkably, the effect of Vacto on the tumor microenvironment is augmented in combination with Pom in the Cereblon-negative immunocompetent 5T33MM model. The phase I data show safety of this agent in combination with Pom. The preliminary efficacy assessment is PFS-6: 100% which is higher than historical controls (Richardson et al. Blood. 2014) with Pom only (PFS-6: 20%) or Pom with corticosteroids (PFS-6:40%). Advancement of Vacto in clinical trial pipelines for MM and other malignant hematological disorders is planned.
Malek:Takeda: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau. Caimi:Kite Pharma: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Celgene: Speakers Bureau; Genentech: Membership on an entity's Board of Directors or advisory committees. Kim:MedPacto: Other: Founder/CEO.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal